







|
NOTICE: The Shelf-Life for this Product Has been Extended to 24 Months, Read the Letter Here. |
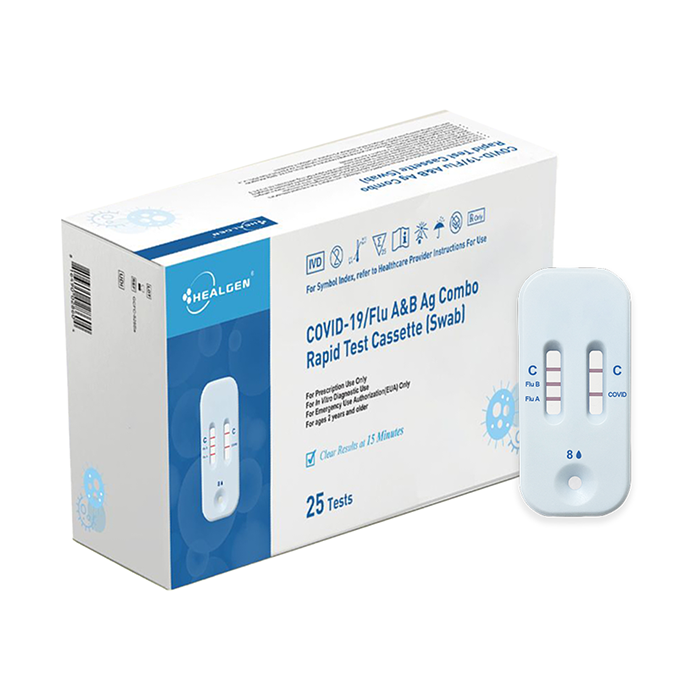
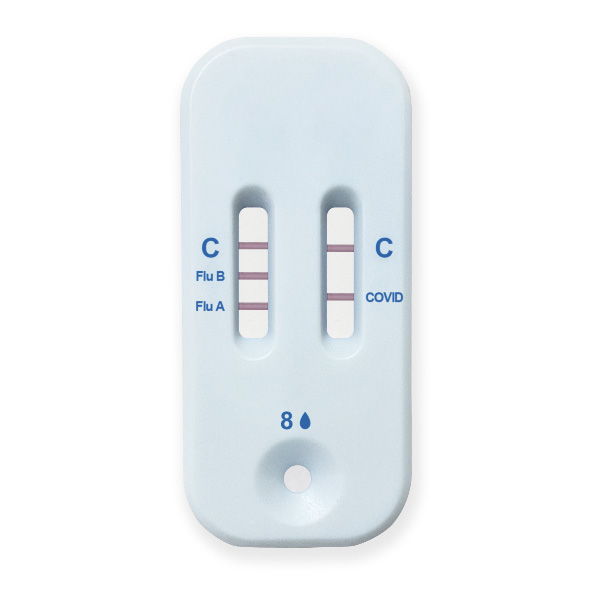
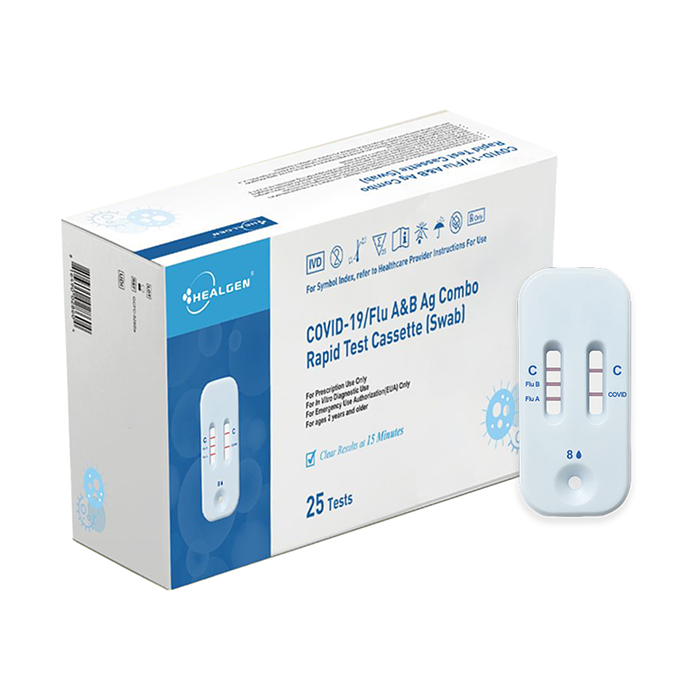
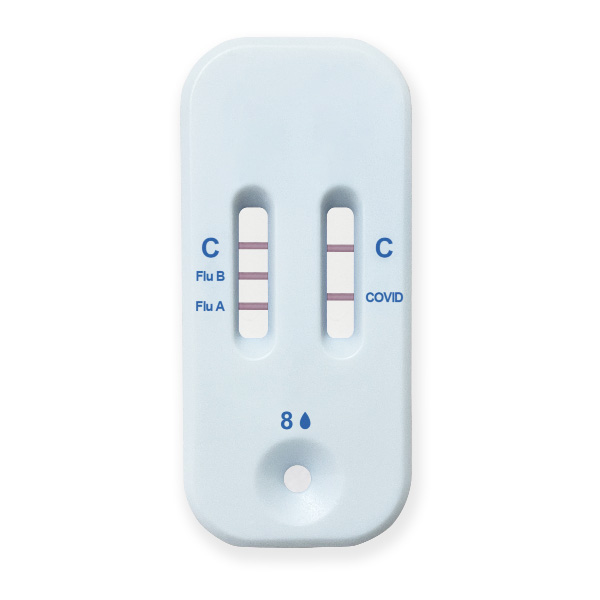
The Healgen® COVID-19/Flu A&B Ag Combo Rapid Test Cassette (swab) is a lateral flow immunochromatographic assay intended for in vitro rapid, simultaneous qualitative detection and differentiation of influenza A and influenza B nucleoprotein antigens and SARS-CoV-2 nucleocapsid antigen directly from anterior nasal swab specimens.
The Healgen® COVID-19/Flu A&B Ag Combo Rapid Test Cassette (swab) is intended for use in individuals who are suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider within the first five (5) days of symptom onset when tested at least twice over three days with at least 48 hours between tests.
Suitable for testing individuals 2+ years old. Swabbing should be performed by an adult for children aged 2 to 13. User must be aged 14 + to perform self test.
Authorized Laboratories: Laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests. This product is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
| Product | Item Code | Quantity |
| COVID-19/Flu A&B Ag Combo Rapid Test Cassette (Swab) | GCFC-525Sa | 25 Tests |
| External control: COVID-19/Flu A&B Ag Combo Rapid Test Cassette (Swab) | GCFC-PN2 | 1 Each negative and positive control swab |
| External control: COVID-19/Flu A&B Ag Combo Rapid Test Cassette (Swab) | GCFC-PN10 | 10 Each negative and positive control swab |
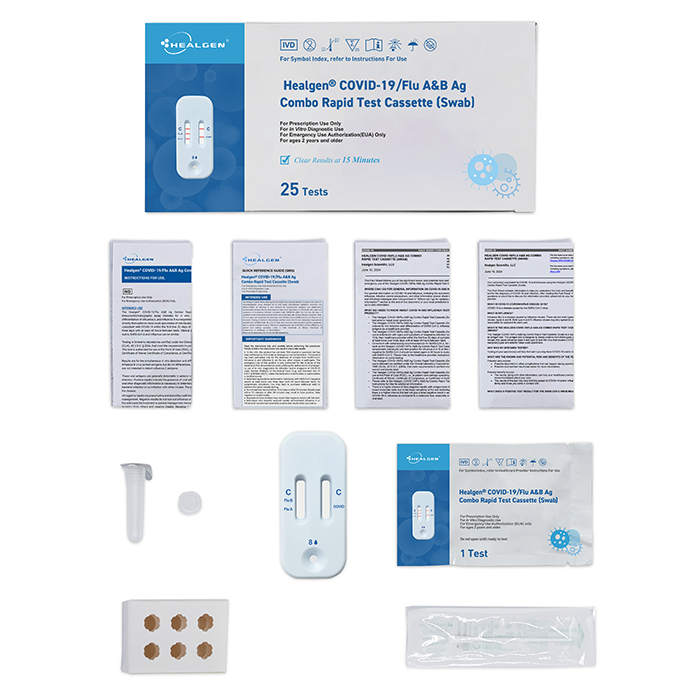
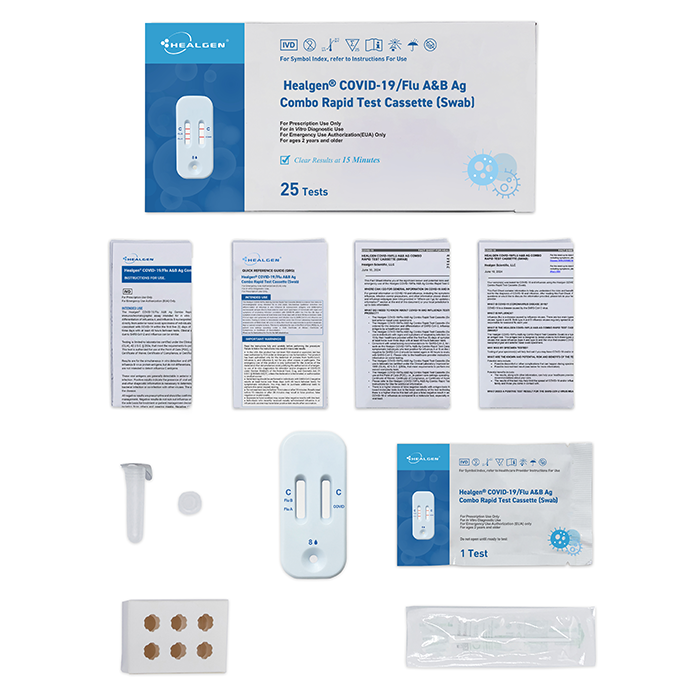
- 25 Sealed Test Cassette
- 25 Sterile Nasal Swabs
- 25 Prefilled Extraction Tube
- 25 Extraction Tube Tip
- 2 Tube Holders
- 1 Package Insert
- 1 Quick Reference Guide (QRG)
|
NOTICE: The Shelf-Life for this Product Has been Extended to 24 Months, Read the Letter Here. |
The Healgen® COVID-19/Flu A&B Ag Combo Rapid Test Cassette (swab) is a lateral flow immunochromatographic assay intended for in vitro rapid, simultaneous qualitative detection and differentiation of influenza A and influenza B nucleoprotein antigens and SARS-CoV-2 nucleocapsid antigen directly from anterior nasal swab specimens.
The Healgen® COVID-19/Flu A&B Ag Combo Rapid Test Cassette (swab) is intended for use in individuals who are suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider within the first five (5) days of symptom onset when tested at least twice over three days with at least 48 hours between tests.
Suitable for testing individuals 2+ years old. Swabbing should be performed by an adult for children aged 2 to 13. User must be aged 14 + to perform self test.
Authorized Laboratories: Laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests. This product is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
| Product | Item Code | Quantity |
| COVID-19/Flu A&B Ag Combo Rapid Test Cassette (Swab) | GCFC-525Sa | 25 Tests |
| External control: COVID-19/Flu A&B Ag Combo Rapid Test Cassette (Swab) | GCFC-PN2 | 1 Each negative and positive control swab |
| External control: COVID-19/Flu A&B Ag Combo Rapid Test Cassette (Swab) | GCFC-PN10 | 10 Each negative and positive control swab |
- The Healgen® COVID-19/Flu A&B Ag Combo Rapid test Cassette (Swab) has not been FDA cleared or approved but has been authorized by FDA under an EUA for use by authorized laboratories.
- This product has been authorized only for the detection of proteins from SARS-CoV-2, influenza A and influenza B, not for any other viruses or pathogens.
- The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
